We owe a lot of great people for their contribution in the discovery of cures and vaccines for many diseases that were the cause of the deaths of many millions of people.
Most of all we owe gratitude to all of the biomedical researchers, those who volunteered for medical trials and to those who gave their lives for the benefit of all humanity. Sometimes, it takes a long time for a person to receive the appropriate affirmation for the global contribution she or he made.
Such is the case with Henrietta Lacks who (unknowingly) gave her cancer cells which went on to become the source of the HeLa cell line – one of the most important experimental cell lines in medical research.
The so-called Hela (Henrietta Lacks) cell line is an immortalized cell line capable of reproducing itself indefinitely if kept under the right conditions.
The first colony of the cells was produced from samples taken from Henrietta’s body without her knowledge. The HeLa cell line is still used in research today, and we have Henrietta to thank for it.

The whole story begins with the unfortunate event of Henrietta being diagnosed with cancer. On January 29, 1951 (soon after she gave birth to her fifth child), Henrietta went to the Johns Hopkins Hospital.
At the time it was the only hospital in the Baltimore, Maryland area that accepted African-American patients. She complained that she was feeling pain in her womb; she felt something like a “knot” inside of her body.
When she went to see her doctor, Howard W. Jones, he recommended that a biopsy should be made of the mass that was growing on Lacks’ cervix. After tests had been made on the sample, Henrietta was told that she had a malignant epidermoid carcinoma of the cervix.
In fact, it was discovered in 1970 that she had been wrongly diagnosed; she actually had an adenocarcinoma. This was a normal mistake during those years and did not adversely affect her treatment as it was the same for both forms of cancer.
After the diagnosis, Henrietta Lacks was held in the hospital for several days and given a treatment of radium tube inserts. After she had been discharged, doctors instructed her to return for follow-up X-ray treatments regularly.
Two of the samples from her cervix that are crucial for this story were taken while Henrietta was laying in the hospital and receiving her treatment. Doctors took one healthy tissue sample and one cancerous sample without her knowledge. The samples were later given to George Otto Gey, a cell biologist and cancer researcher at Johns Hopkins.
In August 1951, 31-year-old Henrietta returned to Johns Hopkins for a routine treatment and complained about very intense abdominal pains. She was admitted to the hospital and given blood transfusions, but her condition deteriorated. Henrietta Lacks died on October 4, 1951, after cancer spread throughout her entire body.
Although Henrietta’s death was tragic, nobody (except the doctors who took her samples and George Otto Gey) knew that a part of her still continued to live. Her cancerous cell sample that eventually became known as the HeLa immortal cell line went on to be the most commonly used cell line in biomedical research.
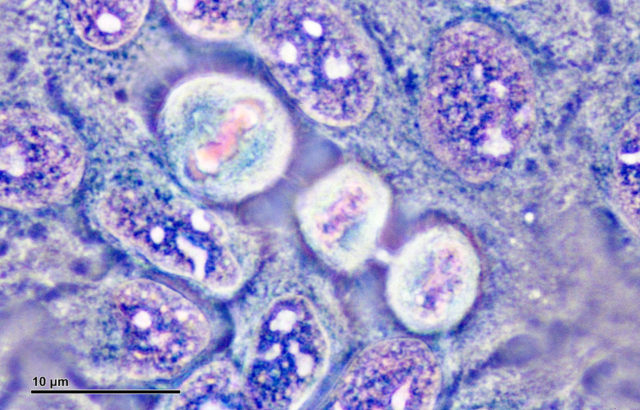
When George Otto Gey put Henrietta’s cancerous cells under the microscope, he noticed something that he hadn’t noticed in any other cell sample before. They reproduced much faster, and he was able to keep them alive longer, which allowed him to do more tests than usual.
Previous cell cultures were active for only a few days, limiting the time available for tests. Lack’s cells were different; they were the first observed cells that divided multiple times without dying and consequently got the name “immortal.” One of Gey’s assistants, Mary Kubicek, even took samples from Henrietta’s dead body while it was still at the Johns Hopkins Hospital.
Soon, Gey managed to start a new cell line from one of the Lack’s samples by isolating one single cell and dividing it continuously. This way, the same cell could be used for doing thousands of experiments. These were the cells that brought a breakthrough in medical research and received the name HeLa cells.
The benefit of the lab-produced HeLa cells was soon very evident. In 1954, when Jonas Salk was developing the polio vaccine, he was using Hela cells to test it.
Chester M. Southam, one of the leading American virologists in the 1950s, used to inject HeLa cells into cancer patients, prison inmates, and healthy persons so that he could test if cancer can be transmitted or if somebody could become immune by developing the necessary antibodies.
Also, HeLa cells were the first human cells to be cloned (1955). Since they were first produced, they have been used in a huge variety of tests and experiments; from testing human sensitivity to tape, glue, cosmetics, and many other products, to research into cancer, AIDS, the effects of radiation and toxic substances, gene mapping, and many other things.

After the 1950s, the demand for HeLa cells became so big that they started to mass produce them. So far, more than 20 tons of Henrietta Lack’s cells have been grown.
All of the experiments continued until the early 1970s when a huge portion of HeLa cells became contaminated by other cell cultures. When that happened, researchers started calling Henrietta Lacks’s family and asking for their blood samples. They wanted to learn more about their genes and try to replace the contaminated Hela cells.
Her family was confused and scared and some of them started to ask questions and demand answers from the people who called them wanting their blood samples. In 1975, during a random dinner party conversation, the family found out what it was all about.
They discovered that samples taken from Henrietta during her time in John Hopkins were still in use for medical research. Faced with this fact, they opened a new chapter in their family history and realized that no one had given permission to Henrietta’s doctors to harvest her cells.
Back in the 1950s, permission for taking samples from somebody’s body was neither required by law or sought by any medical practitioner. That is why nobody knew about Henrietta’s cells being used both for medical research or commercially.
Later, in the 1980s, family medical records were also published without the families knowing about it. When a similar case like Henrietta’s came to the Supreme Court of California, the court ruled that “a person’s discarded tissue and cells are not their property and can be commercialized.”
One of the latest cases of HeLa cells usage without permission happened in March 2013 after a group of researchers published the DNA sequence of the genome of a strain of HeLa cells. When her family heard about this, they immediately objected.
Jeri Lacks Whye, one of Henrietta’s grandchildren, stated her concern in an interview for New York Times:
“the biggest concern was privacy – what information was actually going to be out there about our grandmother, and what information they can obtain from her sequencing that will tell them about her children and grandchildren and going down the line.”

Henrietta’s family struggled for a long time to get justice for the unrightfully obtained samples and, after a long battle, they managed to reach an agreement with the National Institutes of Health in 2013.
The family now has some degree of control over the cell’s DNA sequence and Henrietta’s name will be acknowledged in all scientific papers using HeLa cells. It was also decided that in the future, two people from Henrietta’s family will be part of a six-member committee responsible for regulation of the access to the HeLa sequence data.
Today, the final resting place of the lady that gave so much to the world of medicine is marked with a headstone donated by Roland Pattillo, a colleague of George Gey. The headstone is in the shape of a book, and it contains an epitaph written by her grandchildren.
